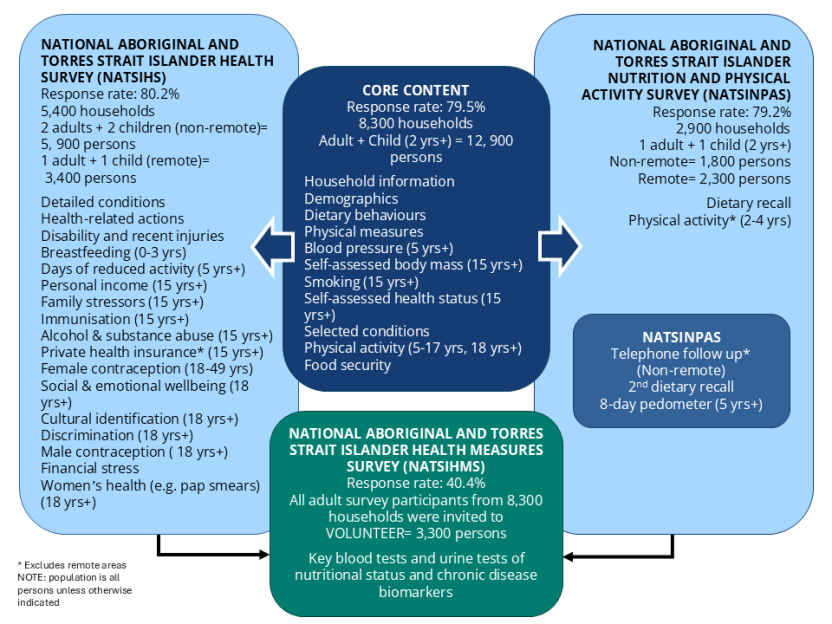
This publication presents information from the National Aboriginal and Torres Strait Islander Health Measures Survey. This survey is the largest biomedical survey ever conducted for Aboriginal and Torres Strait Islander Australians. Around 3,300 Aboriginal and Torres Strait Islander adults (aged 18 years and over) across Australia took part and voluntarily provided blood and/or urine samples, which were tested for a range of chronic disease and nutrient biomarkers.
At the national level, the results showed that:
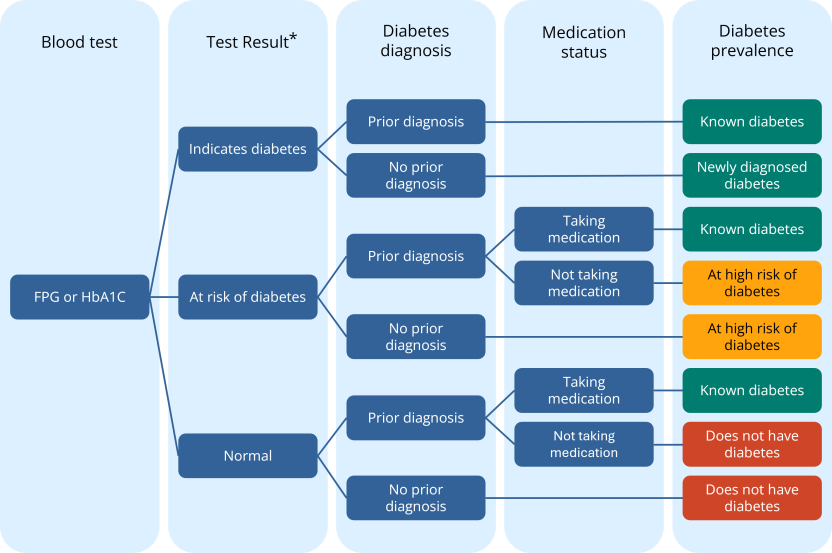
- One in ten (11.1%) Aboriginal and Torres Strait Islander adults had diabetes. This comprised 9.6% with diagnosed diabetes and 1.5% with diabetes newly diagnosed from their test results.
- A further 4.7% were at high risk of diabetes according to their blood test results.
- Two in three (65.3%) had at least one risk factor for cardiovascular disease, that is, they were taking cholesterol-lowering medication or had one or more of high total cholesterol, lower than normal levels of HDL (good) cholesterol, high LDL (bad) cholesterol or high triglycerides.
- Nearly one in five (17.9%) had signs of chronic kidney disease.
It was also revealed that for Aboriginal and Torres Strait Islander adults:
- Around half (53.1%) with diabetes also had signs of chronic kidney disease.
- Two in five (38.9%) with diagnosed diabetes were effectively managing their condition, that is, they had an HbA1c test result of 7.0% or less.
- A quarter (25.0%) had high cholesterol, but only around one in ten (9.1%) of this group were aware they had it.
The survey also found striking differences across remoteness areas. When compared with those in urban areas, Aboriginal and Torres Strait Islander adults in remote areas were:
- Two and a half times as likely to have signs of chronic kidney disease (33.6% compared with 13.1%).
- Around twice as likely to have diabetes (20.8% compared with 9.4%).
- Five times as likely to have newly diagnosed diabetes (4.8% compared with 0.9%).
- Less likely to be effectively managing their diabetes (25.1% compared with 43.5%).
Finally, when compared with the non-Indigenous population (and after adjusting for age differences), Aboriginal and Torres Strait Islander people were:
- More than three times as likely to have diabetes (rate ratio of 3.3).
- Twice as likely to have signs of chronic kidney disease (rate ratio of 2.1).
- Nearly twice as likely to have high triglycerides (rate ratio 1.9).
- More likely to have more than one chronic condition, for example having both diabetes and kidney disease at the same time (53.1% compared with 32.5%).