The potential consequences of the quality incident will influence who needs to be involved in the initial meeting. For example, if the issue has been identified prior to any data being published or released then the issue may be able to be handled at a more local level, with senior managers being kept informed of the issue but not necessarily directly involved in the response. However, if the issue is only identified when the data are already in the public domain, then the QIRP meetings may immediately need to involve the senior management of the organisation, as the potential consequences to the reputation of the organisation and impact of incorrect data in the public domain will need to be managed quickly.
Who to include in the initial meeting will depend on the nature of quality incident. Initially, it is probably best to include more people rather than less to ensure that all the issues and potential solutions are identified. Participants at the initial meeting would include those people who are directly responsible for producing the statistics and those people who support the production of the statistics. For example, at the ABS not only would the collection area people be involved, but people from the methodology area and time series analysis area (if relevant) would be involved in the meeting. Other participants might include areas of the ABS who are responsible for other aspects of the collection cycle that may impact on the statistics and process in question. Once again, participants at the meeting will depend on the nature of the quality incident and where it has been identified in the collection cycle process.
Other stakeholders who may be kept informed of progress but do not attend the meeting include areas of the organisation who are not directly involved in the production of the statistics but their work is dependent upon them. For example, at the ABS the publishing area who manages the ABS website and its releases would be kept informed of any potential delays to statistical releases in order to manage their workload accordingly.
Along with identifying the participants, expectations about resourcing also need to be stated. Participating stakeholders need to be aware that they may be expected to allocate dedicated resources to the QIRP process until the issue is resolved.
It is important that all the key people hear all the facts first hand. Once the QIRP is underway there is no time for misunderstandings about what is needed, why, or what it might mean at a later date. It helps if everyone involved is aware of the entire process and their part in it so that they can adapt and help each other. Having all the stakeholders together initially is a more effective way of managing the QIRP compared to having multiple bilateral meetings. The identifying of all of the issues, understanding the problem, working out potential solutions, identifying implications and dependencies, and agreeing on an action plan in a very short period of time is facilitated through having all key players together at the first QIRP meeting.
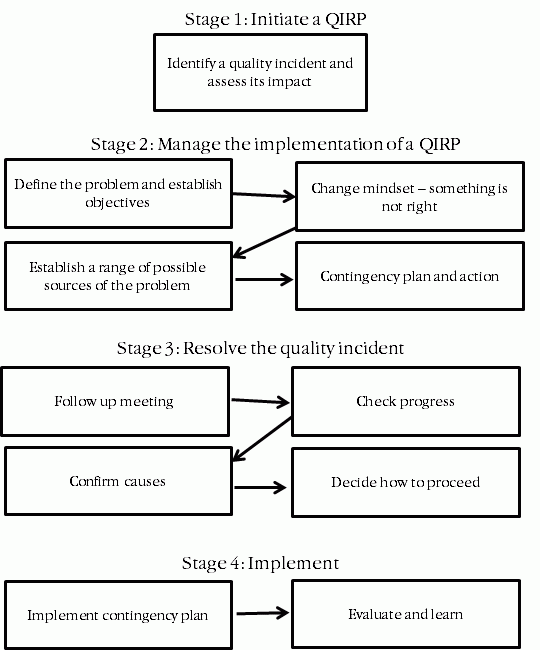
To effectively manage the QIRP, roles for addressing the quality incident need to be defined up front to ensure that stakeholders are aware of their responsibilities and time is not wasted due to further confusion. The roles that need to be established in this step are:
- A facilitator: It may be useful to have an independent facilitator for the fact gathering process to allow those involved in the investigation process to participate fully. The tasks of the facilitator in the initial and any subsequent meetings would be to draw out all of the potentially relevant facts, to keep the meetings on track, and to ensure that all participants are engaged and consulted. This ensures all relevant information is considered for resolving the quality incident.
- A driver: A driver is the person responsible for driving and coordinating the process from start to finish. Their role includes progressing issues, being aware of who is doing what and when, and documenting the process and the actions to be carried out with the assistance of a note taker. The driver is also responsible for troubleshooting business as usual obstacles that may arise during the process to ensure that the incident is resolved within a short time frame. As part of their role they are also prepared to drop everything else in order to focus on the QIRP.
- A meeting co-ordinator: A meeting co-ordinator is responsible for determining who should be included in the QIRP meetings, scheduling the meetings and circulating agendas.
- A note taker: The note taker is responsible for recording the key facts and points of discussion and passing these notes on to the meeting co-ordinator for circulation post meeting.
- Quality incident investigators: The initial QIRP meeting will typically establish a range of investigations that may be required to find the source of the quality incident. The people responsible for carrying out these investigations and reporting back to the QIRP driver need to be aware of their role and the responsibilities associated with that role.
Note that different people are not necessarily required for each of the roles. For example, the driver and meeting co-ordinator may be the same person. However, it is important to have each of these roles identified and allocated so that the underlying responsibilities are not missed.